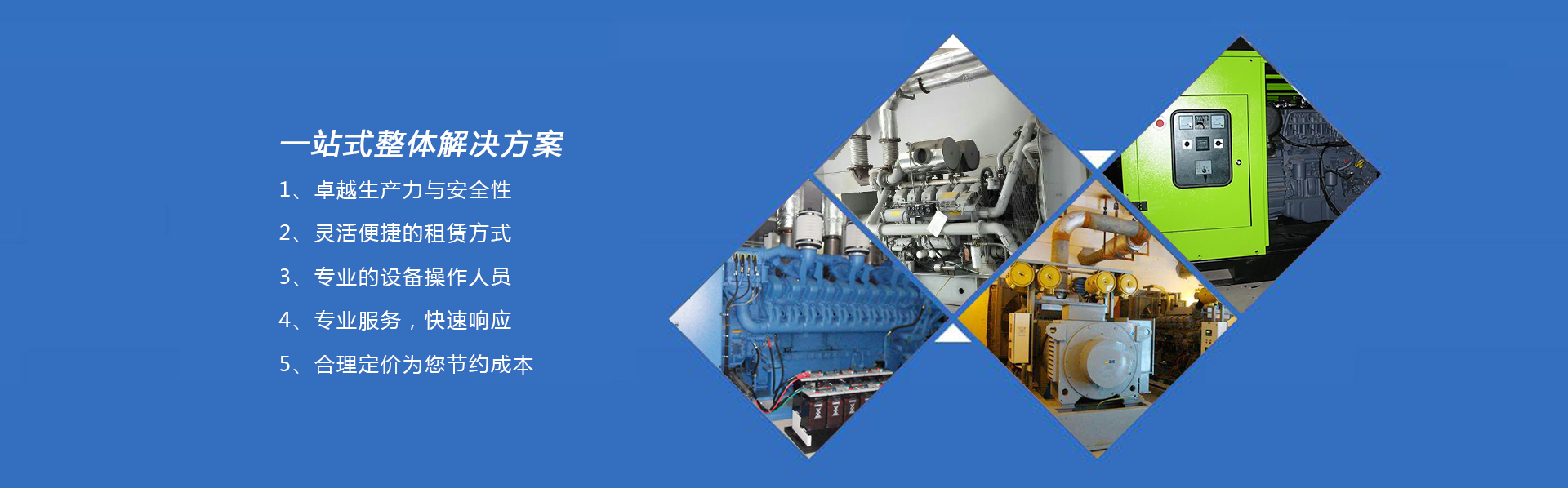
產(chǎn)品
聯(lián)系我們
蘇州工業(yè)園區(qū)利通機(jī)電服務(wù)有限公司
地址:蘇州市吳中經(jīng)濟(jì)開(kāi)發(fā)區(qū)前珠路16號(hào)
電話(huà):0512-66258344
傳真:0512-66258344
郵箱:zxmhike@163.com
地址:蘇州市吳中經(jīng)濟(jì)開(kāi)發(fā)區(qū)前珠路16號(hào)
電話(huà):0512-66258344
傳真:0512-66258344
郵箱:zxmhike@163.com
產(chǎn)品目錄